PLEASE HELP WILL GIVE
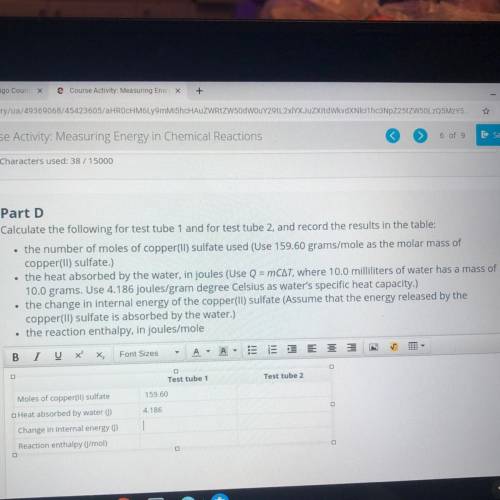
Part D
Calculate the following for test tube 1 and for test tube 2, and...

PLEASE HELP WILL GIVE
Part D
Calculate the following for test tube 1 and for test tube 2, and record the results in the table:
• the number of moles of copper(II) sulfate used (Use 159.60 grams/mole as the molar mass of
copper(ll) sulfate.)
• the heat absorbed by the water, in joules (Use Q = mCat, where 10,0 milliliters of water has a mass of
10,0 grams. Use 4.186 Joules/gram degree Celsius as water's specific heat capacity.)
• the change in internal energy of the copper(II) sulfate (Assume that the energy released by the
copper(ll) sulfate is absorbed by the water.)
• the reaction enthalpy, in joules/mole

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Which of the following statements is true? question 4 options: nuclear decay rates vary with the conditions of the reaction, but chemical reaction rates do not. chemical reaction rates vary with the conditions of the reaction, but nuclear decay rates do not. neither chemical reaction rates nor nuclear decay rates vary with the conditions of the reaction. both chemical reaction rates and nuclear decay rates vary with the conditions of the reaction.
Answers: 1

Chemistry, 21.06.2019 18:00
When the following equation is balanced using the smallest possible integers, what is the coefficent of oxygen gas? c7h16o(g) + o2(g) → co2(g) + h2o(g) -1 -5 -8 -16 -21
Answers: 3

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

You know the right answer?
Questions



Advanced Placement (AP), 05.01.2020 06:31



History, 05.01.2020 06:31



Mathematics, 05.01.2020 06:31


English, 05.01.2020 06:31



English, 05.01.2020 06:31

Mathematics, 05.01.2020 06:31

Mathematics, 05.01.2020 06:31

History, 05.01.2020 06:31


Health, 05.01.2020 06:31



