
Chemistry, 26.05.2021 18:30 renegade2020
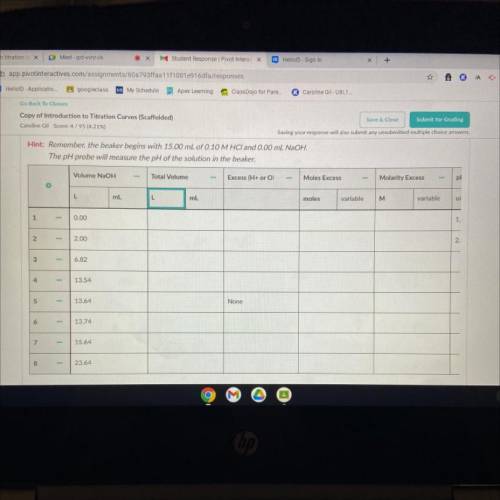
For each volume NaOH, determine the following and record in the data table.
1. Total volume of solution after NaOH is added. (The beaker starts with 15.00 mL HCI)
2. The ion in excess. There is no excess ion at the equivalence point, so leave it blank at that volume.
3. The moles excess reactant (use stoichiometry).
4. The molarity of excess reactant.
5. pH of solution

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
An electrons position cannot be known precisely only it's probability of being in a certain location can be known
Answers: 1

Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 17:30
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2

You know the right answer?
For each volume NaOH, determine the following and record in the data table.
1. Total volume of solu...
Questions


Geography, 25.08.2019 14:50

Biology, 25.08.2019 14:50

Social Studies, 25.08.2019 14:50

History, 25.08.2019 14:50





Mathematics, 25.08.2019 14:50

Mathematics, 25.08.2019 14:50

Spanish, 25.08.2019 14:50



Biology, 25.08.2019 14:50


Biology, 25.08.2019 14:50

Chemistry, 25.08.2019 14:50

History, 25.08.2019 14:50




