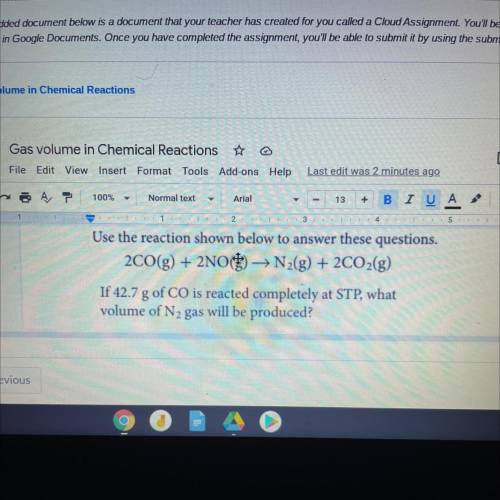
Use the reaction shown l to answer these questions.
2CO(g) + 2NO) → N2(g) + 2CO2(g)
If 42.7 g...

Chemistry, 26.05.2021 19:00 berlyntyler
Use the reaction shown l to answer these questions.
2CO(g) + 2NO) → N2(g) + 2CO2(g)
If 42.7 g of CO is reacted completely at STP, what
volume of N2 gas will be produced?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 20:00
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1

Chemistry, 22.06.2019 20:30
Some familiar products contain some of the same types of atoms. for instance, the chemical formula for baking soda is nahco 3. the chemical formula for liquid bleach is naclo, and the chemical formula for table salt is nacl. which choice best describes why these three products have some of the same types of atoms in common?
Answers: 1

Chemistry, 23.06.2019 01:30
What is produced from neutralization of an acid and a base? a. hydronium ions b. citric acid c. salt and water
Answers: 1
You know the right answer?
Questions


Mathematics, 16.12.2020 19:30


Biology, 16.12.2020 19:30

English, 16.12.2020 19:30

Mathematics, 16.12.2020 19:30


Mathematics, 16.12.2020 19:30




Mathematics, 16.12.2020 19:30




Mathematics, 16.12.2020 19:30

English, 16.12.2020 19:30

Mathematics, 16.12.2020 19:30


English, 16.12.2020 19:30



