
Chemistry, 26.05.2021 19:00 kamster911
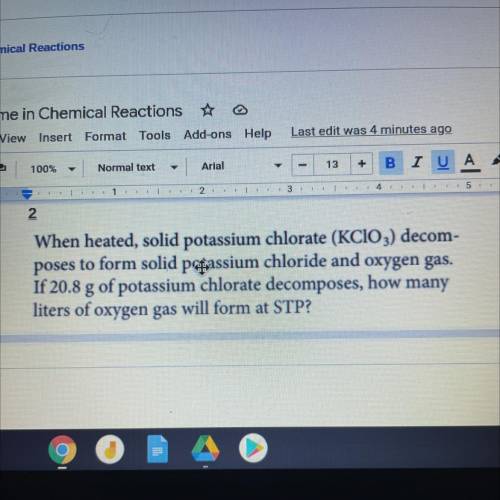
When heated, solid potassium chlorate (KClO3) decom-
poses to form solid passium chloride and oxygen gas.
If 20.8 g of potassium chlorate decomposes, how many
liters of oxygen gas will form at STP?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 12:00
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 17:30
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1
You know the right answer?
When heated, solid potassium chlorate (KClO3) decom-
poses to form solid passium chloride and oxyge...
Questions


Mathematics, 16.01.2020 01:31




Biology, 16.01.2020 01:31

Mathematics, 16.01.2020 01:31

Mathematics, 16.01.2020 01:31



History, 16.01.2020 01:31



Mathematics, 16.01.2020 01:31


Biology, 16.01.2020 01:31

Mathematics, 16.01.2020 01:31

English, 16.01.2020 01:31




