
Chemistry, 26.05.2021 19:20 trevorhenyan51
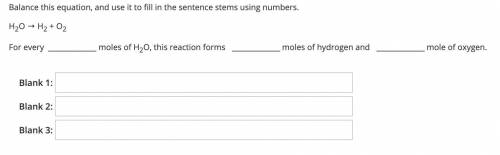
H2O → H2 + O2
For every moles of H2O, this reaction forms moles of hydrogen and mole of oxygen.
Blank 1:
Blank 2:
Blank 3:
PLEASE HELP

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1

Chemistry, 22.06.2019 22:30
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1

Chemistry, 23.06.2019 01:00
Which substance—wood or silver—is the better thermal conductor? a thermal conductor is a material that requires very little heat energy to change its temperature. explain your answer.
Answers: 3
You know the right answer?
H2O → H2 + O2
For every moles of H2O, this reaction forms moles of hydrogen and mole of oxygen.
Questions

Mathematics, 03.05.2021 16:50

Computers and Technology, 03.05.2021 16:50

Mathematics, 03.05.2021 16:50

Advanced Placement (AP), 03.05.2021 16:50

Biology, 03.05.2021 16:50

Social Studies, 03.05.2021 16:50


Mathematics, 03.05.2021 16:50




History, 03.05.2021 16:50

Social Studies, 03.05.2021 16:50

Law, 03.05.2021 16:50

Mathematics, 03.05.2021 16:50

Mathematics, 03.05.2021 16:50



Mathematics, 03.05.2021 16:50



