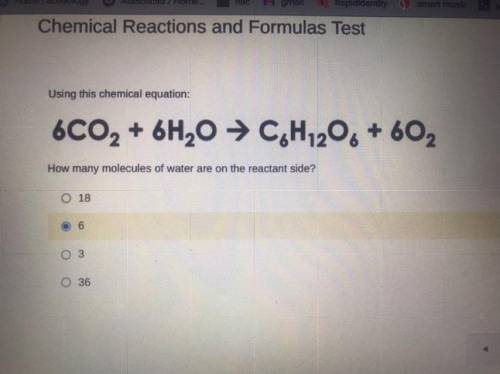
Using this chemical equation:
6CO2 + 6H2O → C, H,206 + 602
How many molecules of water are on...

Chemistry, 26.05.2021 20:30 wanderlay29
Using this chemical equation:
6CO2 + 6H2O → C, H,206 + 602
How many molecules of water are on the reactant side?. HELP DUE IN 20 MINUTES

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 08:30
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2

Chemistry, 22.06.2019 13:00
Lab reagent, hypothesis test.a reference solution used as a lab reagent is purported to have a concentration of 5 mg/dl. six samples are taken from this solution and the following concentrations are recorded: (5.32, 4.88, 5.10, 4.73, 5.15, 4.75) mg/dl.these six measurements are assumed to be an srs of all possible measurements from solution.they are also assumed to have a standard deviation of 0.2, a normal distributin, and a mean concentration equal to the true concentration of the solution.carry out a significance test to determine whether these six measurements provide reliable evidence that the true concentration of the solution is actually not 5 mg/dl.
Answers: 1
You know the right answer?
Questions

Chemistry, 16.12.2020 17:00




English, 16.12.2020 17:00

English, 16.12.2020 17:00

Computers and Technology, 16.12.2020 17:00

Mathematics, 16.12.2020 17:00















