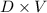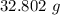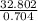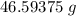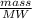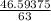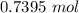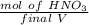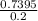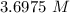Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the formula that this ionic compounds could form sr2+p3-o2-
Answers: 3

Chemistry, 22.06.2019 07:30
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3

Chemistry, 22.06.2019 07:30
What is i fracture in the crust called when land move up, down or sideways
Answers: 2

Chemistry, 22.06.2019 17:30
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2
You know the right answer?
A solution is made by adding 23.1 mL of concentrated nitric acid ( 70.4 wt% , density 1.42 g/mL ) to...
Questions

Mathematics, 14.09.2020 03:01

History, 14.09.2020 03:01

Mathematics, 14.09.2020 03:01

History, 14.09.2020 03:01

Mathematics, 14.09.2020 03:01

Mathematics, 14.09.2020 03:01

French, 14.09.2020 03:01

History, 14.09.2020 03:01

Mathematics, 14.09.2020 03:01

Chemistry, 14.09.2020 03:01

Mathematics, 14.09.2020 03:01

Mathematics, 14.09.2020 03:01

History, 14.09.2020 03:01

Mathematics, 14.09.2020 03:01

English, 14.09.2020 03:01

Mathematics, 14.09.2020 03:01

Physics, 14.09.2020 03:01

Mathematics, 14.09.2020 03:01

History, 14.09.2020 03:01

Mathematics, 14.09.2020 03:01