
Chemistry, 27.05.2021 05:20 wrightlilybug07
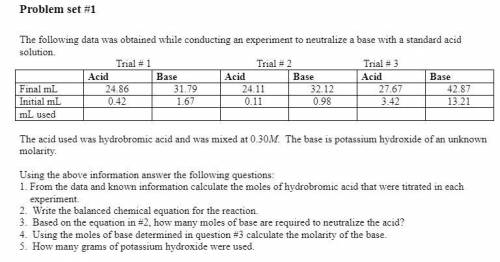
Using the above information answer the following questions:
1. From the data and known information calculate the moles of hydrobromic acid that were titrated in each
experiment.
2. Write the balanced chemical equation for the reaction.
3. Based on the equation in #2, how many moles of base are required to neutralize the acid?
4. Using the moles of base determined in question #3 calculate the molarity of the base.
5. How many grams of potassium hydroxide were used.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change. when the temperature in a room increases from 25°c to 33°c, changes from a solid to a liquid. in a lab, methane and nitrogen are cooled from -170°c to -200°c. the methane freezes and the nitrogen . when gold is heated to 2,856°c it changes from a liquid to a .
Answers: 2


Chemistry, 22.06.2019 14:30
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1

Chemistry, 23.06.2019 01:00
Which polymers are most closely related? a. protein and nucleic acids b. cellulose and starch c. nucleic acids and starch d. nucleic acids and cellulose
Answers: 2
You know the right answer?
Using the above information answer the following questions:
1. From the data and known information...
Questions


Mathematics, 05.01.2021 22:50


Mathematics, 05.01.2021 22:50

English, 05.01.2021 22:50

Mathematics, 05.01.2021 22:50




Mathematics, 05.01.2021 23:00

History, 05.01.2021 23:00

Computers and Technology, 05.01.2021 23:00

English, 05.01.2021 23:00


Social Studies, 05.01.2021 23:00





Mathematics, 05.01.2021 23:00



