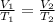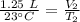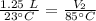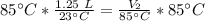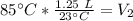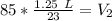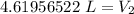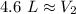Chemistry, 27.05.2021 06:40 carolinamleal04
A balloon containing helium gas has a volume of 1.25 L at room temperature (23 oC). The balloon is heated to at temperature of 85 oC . Assuming no change in pressure, what is the new volume of the balloon?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2


Chemistry, 23.06.2019 02:30
Which statement best describes the liquid state of matter? a. it has definite shape but indefinite volume. b. it has definite shape and definite volume. c. it has indefinite shape and indefinite volume. d. it has indefinite shape but definite volume.
Answers: 1

Chemistry, 23.06.2019 06:00
What are the coefficients to balance the following equation? ba+br2=babr2
Answers: 2
You know the right answer?
A balloon containing helium gas has a volume of 1.25 L at room temperature (23 oC). The balloon is h...
Questions




Mathematics, 12.05.2021 08:30



English, 12.05.2021 08:30

Mathematics, 12.05.2021 08:30

Computers and Technology, 12.05.2021 08:30

Mathematics, 12.05.2021 08:30

Mathematics, 12.05.2021 08:30


Business, 12.05.2021 08:30

Computers and Technology, 12.05.2021 08:30

Mathematics, 12.05.2021 08:30

English, 12.05.2021 08:30

English, 12.05.2021 08:30

Mathematics, 12.05.2021 08:30

Mathematics, 12.05.2021 08:30

Spanish, 12.05.2021 08:30