Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1


Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3

Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1
You know the right answer?
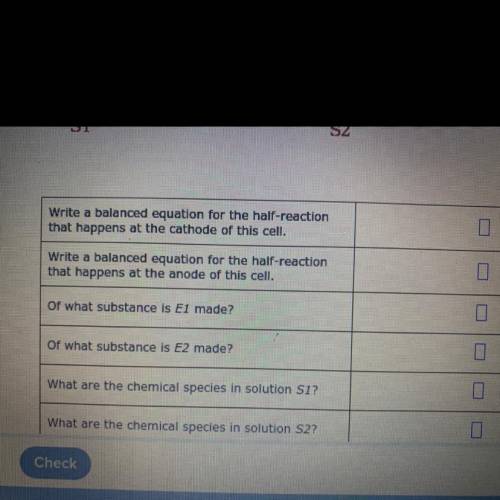
Suppose the galvanic cell sketched below is powered by the following reaction:
Mg(s)+Zn(NO3)2(aq) →...
Questions



Mathematics, 18.06.2020 16:57

Mathematics, 18.06.2020 16:57


English, 18.06.2020 16:57





English, 18.06.2020 16:57

Mathematics, 18.06.2020 16:57

History, 18.06.2020 16:57




Mathematics, 18.06.2020 16:57

Mathematics, 18.06.2020 16:57