Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2

Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1
You know the right answer?
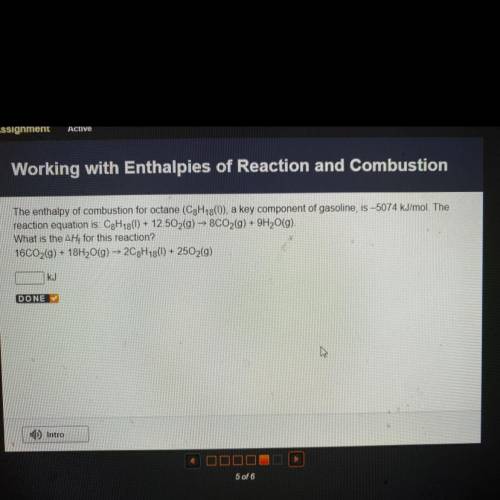
The enthalpy of combustion for octane (C3H13()), a key component of gasoline, is –5074 kJ/mol. The...
Questions

Mathematics, 18.08.2019 16:30

Social Studies, 18.08.2019 16:30

Social Studies, 18.08.2019 16:30

Mathematics, 18.08.2019 16:30

Mathematics, 18.08.2019 16:30

Biology, 18.08.2019 16:30



Geography, 18.08.2019 16:30

English, 18.08.2019 16:30

Mathematics, 18.08.2019 16:30

History, 18.08.2019 16:30

Social Studies, 18.08.2019 16:30

Chemistry, 18.08.2019 16:30




Mathematics, 18.08.2019 16:30

Biology, 18.08.2019 16:30

Computers and Technology, 18.08.2019 16:30