
Chemistry, 27.05.2021 21:30 moneybabyy38
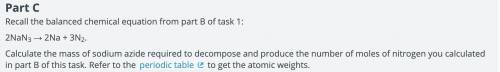
One of the main components of an airbag is the gas that fills it. As part of the design process, you need to determine the exact amount of nitrogen that should be produced. Calculate the number of moles of nitrogen required to fill the airbag. Show your work. Assume that the nitrogen produced by the chemical reaction is at a temperature of 495°C and that nitrogen gas behaves like an ideal gas.


Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 22:00
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3

Chemistry, 23.06.2019 07:00
The following transition occurs at a molecular level for a substance. what transition corresponds to this change in microscopic structure? the carbon dioxide molecules on the left are in a regular, tightly packed pattern. after heating, it becomes much lower density. a. melting b. boiling c. sublimation d. freezing
Answers: 1

Chemistry, 24.06.2019 01:00
What is an effect of gravity on objects on the surface of earth? check all that apply. objects “fall” toward the center of earth. objects are pulled by earth but do not pull on earth. objects accelerate at a rate of 9.8 m/s each second. objects travel at a rate of 9.8 m/s toward the center of earth. objects are pulled by earth more strongly than they pull on earth.
Answers: 1
You know the right answer?
One of the main components of an airbag is the gas that fills it. As part of the design process, you...
Questions

Mathematics, 20.04.2020 13:50

Mathematics, 20.04.2020 13:50


Mathematics, 20.04.2020 13:50


History, 20.04.2020 13:50

Chemistry, 20.04.2020 13:50


Mathematics, 20.04.2020 13:50


History, 20.04.2020 13:50

Health, 20.04.2020 13:50

English, 20.04.2020 13:50

Mathematics, 20.04.2020 13:51


English, 20.04.2020 13:51

History, 20.04.2020 13:51





