
Chemistry, 27.05.2021 21:40 sammizwang
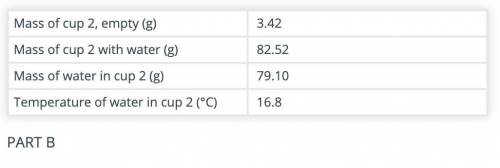
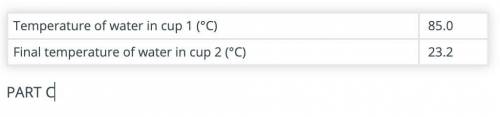
Calculate the amount of heat gained by the water in cup 2 after adding the hot object(s) to it.
Use the data recorded in parts B and C and the formula Q = mCΔT. The specific heat capacity of water is 4.186 J/(g °C).
(part B and C numbers are included)

Answers: 1


Another question on Chemistry

Chemistry, 20.06.2019 18:04
Brad and his family like to go camping. they always take a gas lantern with them. the lantern can take fiuels but always puts out the same amount of light. brad has two different kinds of fuel for the lantern. how can he figure out which kind of fuel has more chemical energy that the lantern can turn into light? measure the brightness of the lantern using each kind of fuel by comparing it to the full moon. g measure the volume of the containers of fuel. measure how long the lantern stays lit on a fixed amount of fuel. measure the mass of each container of fuel using a balance scale. answer 12
Answers: 1

Chemistry, 22.06.2019 02:30
Margaret wants to make an orange flavored drink by stirring powdered drink mix into a glass of water. she doesn't like drinks that have small clumps of powdered solid in them, so she wants the drink to be a perfect solution. what factors should margaret not consider when deciding how much powder to add to her glass of water?
Answers: 3


Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1
You know the right answer?
Calculate the amount of heat gained by the water in cup 2 after adding the hot object(s) to it.
Use...
Questions



History, 16.07.2019 22:30





Mathematics, 16.07.2019 22:30

Social Studies, 16.07.2019 22:30


Mathematics, 16.07.2019 22:30

Biology, 16.07.2019 22:30

History, 16.07.2019 22:30


Mathematics, 16.07.2019 22:30


Arts, 16.07.2019 22:30



Mathematics, 16.07.2019 22:30



