Chemistry, 27.05.2021 22:50 rachellynn02
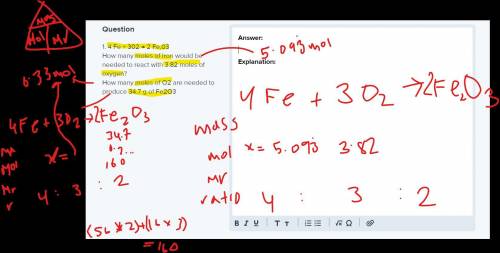
1. 4 Fe + 302 → 2 Fe,03
How many moles of iron would be needed to react with 3.82 moles of oxygen?
How many moles of O2 are needed to produce 34.7 g of Fe2O3

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1

Chemistry, 22.06.2019 07:20
2pos suppose an object in free fall is dropped from a building. its starting velocity is 0 m/s. ignoring the effects of air resistance, what is the speed (in m/s) of the object after falling 3 seconds? give your answer as a positive decimal without units. answer here
Answers: 1

Chemistry, 22.06.2019 09:00
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1

Chemistry, 23.06.2019 07:20
Which of the following are acids or bases? 1. sodium hydrogen 2. barium hydroxide solution 3. carbonate solution
Answers: 1
You know the right answer?
1. 4 Fe + 302 → 2 Fe,03
How many moles of iron would be needed to react with 3.82 moles of oxygen?<...
Questions

Mathematics, 22.02.2021 23:20


Mathematics, 22.02.2021 23:20

English, 22.02.2021 23:20




Mathematics, 22.02.2021 23:20


Mathematics, 22.02.2021 23:20



Biology, 22.02.2021 23:20

Mathematics, 22.02.2021 23:20


History, 22.02.2021 23:20


Mathematics, 22.02.2021 23:20

Mathematics, 22.02.2021 23:20

Mathematics, 22.02.2021 23:20