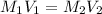Chemistry, 28.05.2021 01:00 donaldwilliams31
Describe how you would make 800.0 mL of a 0.400 M NaOH solution from a 17.0 M stock NaOH solution. Express your answer with the appropriate units.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Liv sheldon given the balanced equation for an organic reaction: c2h2 + 2cl2 → c2h2cl4 this reaction is best classified as *
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 17:00
The biosphere of the earth is made up of what compound? organic or inorganic?
Answers: 3

Chemistry, 22.06.2019 20:50
What is the vapor pressure of a solution with a benzene to octane?
Answers: 2
You know the right answer?
Describe how you would make 800.0 mL of a 0.400 M NaOH solution from a 17.0 M stock NaOH solution. E...
Questions

Mathematics, 12.08.2020 06:01

Mathematics, 12.08.2020 06:01

Mathematics, 12.08.2020 06:01

Mathematics, 12.08.2020 06:01


English, 12.08.2020 06:01



Biology, 12.08.2020 06:01

History, 12.08.2020 06:01

Mathematics, 12.08.2020 06:01



Mathematics, 12.08.2020 06:01


History, 12.08.2020 06:01



Mathematics, 12.08.2020 06:01