
Chemistry, 28.05.2021 04:20 brillamontijo
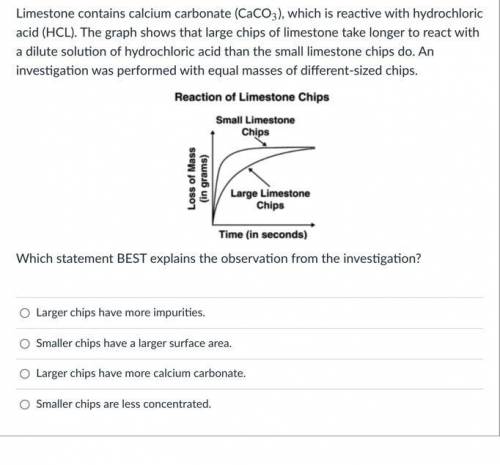
Limestone contains calcium carbonate (CaCO3), which is reactive with hydrochloric acid (HCL). The graph shows that large chips of limestone take longer to react with a dilute solution of hydrochloric acid than the small limestone chips do. An investigation was performed with equal masses of different-sized chips.
Which statement BEST explains the observation from the investigation?
Group of answer choices
Larger chips have more impurities.
Smaller chips have a larger surface area.
Larger chips have more calcium carbonate.
Smaller chips are less concentrated.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1

Chemistry, 22.06.2019 21:00
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1

You know the right answer?
Limestone contains calcium carbonate (CaCO3), which is reactive with hydrochloric acid (HCL). The gr...
Questions




Mathematics, 27.09.2021 07:40

History, 27.09.2021 07:40


Mathematics, 27.09.2021 07:40

History, 27.09.2021 07:40




Biology, 27.09.2021 07:40

Mathematics, 27.09.2021 07:40


Mathematics, 27.09.2021 07:40







