
Chemistry, 28.05.2021 20:00 pedropaulofpedrosapp
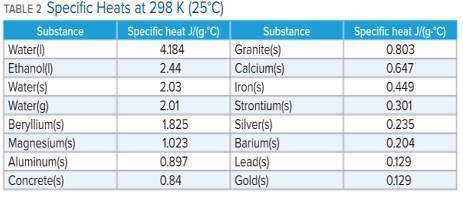
A 136 g sample of an unknown substance was heated from 20.0 °C to 40.0 °C. In the process the substance absorbed 2440 J of energy. What is the specific heat of the substance? Identify the substance among those listed in Table 2.
A. the specific heat is 0.897 J/g. C, The Substance is aluminum
B. the specific heat is -0.897 J/g. C, The Substance is aluminum
C. the specific heat is 4.184 J/g. C, The Substance is water
D. there's not enough information to determine which is the substance.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
If i make a solution by adding 83grams of sodium hydroxide to 750ml i’d water what is the molarity of sodium hydroxide
Answers: 1

Chemistry, 22.06.2019 06:30
The best solution for preventing harm to people and pets from severe hurricanes involves determining and warning residents about what
Answers: 1

Chemistry, 22.06.2019 07:30
Free answer. the treaty of versailles ended world war i, but some of the terms of the treaty contributed to the beginning of world war ii. which was one of the terms of the treaty? the answer would be "germany was forces to pay reparations to the allied countries.". i hope this .
Answers: 1

Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1
You know the right answer?
A 136 g sample of an unknown substance was heated from 20.0 °C to 40.0 °C. In the process the substa...
Questions




Mathematics, 15.12.2021 04:10




Mathematics, 15.12.2021 04:10

Social Studies, 15.12.2021 04:10


Advanced Placement (AP), 15.12.2021 04:10

Mathematics, 15.12.2021 04:10


Physics, 15.12.2021 04:10

Chemistry, 15.12.2021 04:10



Biology, 15.12.2021 04:10

English, 15.12.2021 04:10

Chemistry, 15.12.2021 04:10



