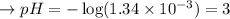Chemistry, 29.05.2021 01:40 Queenbee2304
Calculate the pH of a 0.10 M solution of acidic acid HC2H3O2(aq) at 25 °C. Ka for HC2H3O2 = 1.8×10−5 at 25 °C g

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:50
Problem page gaseous ethane reacts with gaseous oxygen gas to produce gaseous carbon dioxide and gaseous water . if of water is produced from the reaction of of ethane and of oxygen gas, calculate the percent yield of water. be sure your answer has the correct number of significant digits in it.
Answers: 2

Chemistry, 21.06.2019 20:30
14. complete and balance the equations for the single displacement reactions. a. zn + pb(no3)2 -> b. al + niso4 -> 15. complete and balance the equations for the double displacement reactions. a. agno3(aq) + nacl(aq) -> b. mg(no3)2(aq) + koh(aq) -> 16. complete and balance the equations for the combustion reactions. a. __ ch4 + o2 -> b. __ c3h6 + o2 -> c. + o2 ->
Answers: 2


Chemistry, 21.06.2019 23:00
At room temperature what happens to the average kinetic energy of the molecules of a solid, liquid, and a gas
Answers: 2
You know the right answer?
Calculate the pH of a 0.10 M solution of acidic acid HC2H3O2(aq) at 25 °C. Ka for HC2H3O2 = 1.8×10−5...
Questions

Mathematics, 03.11.2019 09:31


History, 03.11.2019 09:31



Mathematics, 03.11.2019 09:31


Computers and Technology, 03.11.2019 09:31

Health, 03.11.2019 09:31

Chemistry, 03.11.2019 09:31




English, 03.11.2019 09:31

Biology, 03.11.2019 09:31

Advanced Placement (AP), 03.11.2019 09:31

Mathematics, 03.11.2019 09:31


Mathematics, 03.11.2019 09:31

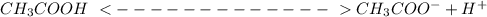
![Ka = \frac{[CH3COO^{-}] [H^{+}]}{[CH_3COOH]}\\\\](/tpl/images/1354/5896/b6175.png)
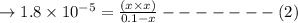
![1.8\times 10^{-5} = \frac{x^2}{0.10}\\\\x^2 = 1.8 \times 10^{-6}\\\\x = 1.34 \times 10^{-3}\\\\pH = -\log [H^{+}]](/tpl/images/1354/5896/b9098.png)
![[H^{+}] = x = 1.34 \times 10^{-3}](/tpl/images/1354/5896/38a1b.png)