
Chemistry, 29.05.2021 03:20 corlissquillen13
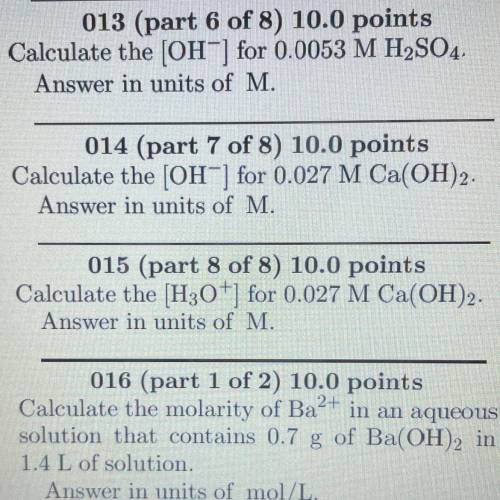
I need help with 13 and 15 please‼️‼️‼️ASAP‼️‼️‼️
will give brainliest if correct

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
You encounter a solution that is acidic and you decide to test it by adding a small amount of a strong acid. the ph lowers slightly but is approximately unchanged, and still remains acidic. what can you say about the solution? a. it is a buffer solution. b. it is not a buffer solution it is a strong acid solution. d. the solution has been neutralized. e. the solution has excess acid present
Answers: 1

Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1

Chemistry, 22.06.2019 23:00
What is the oxidation state of each individual carbon atom in c2o42−?
Answers: 1
You know the right answer?
I need help with 13 and 15 please‼️‼️‼️ASAP‼️‼️‼️
will give brainliest if correct
...
will give brainliest if correct
...
Questions

English, 28.08.2021 04:40


Mathematics, 28.08.2021 04:40

Mathematics, 28.08.2021 04:40


History, 28.08.2021 04:40

Mathematics, 28.08.2021 04:40


Mathematics, 28.08.2021 04:40

Mathematics, 28.08.2021 04:40



Mathematics, 28.08.2021 04:40







Mathematics, 28.08.2021 04:40



