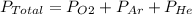Chemistry, 29.05.2021 04:30 bacchus2847
The total pressure of an O2-Ar-He gas mixture is 43 atm. If the partial pressure of Ar is 15 atm and the partial pressure of He is 7 atm, then the partial pressure of O2 is -

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 00:30
There are approximately 15 milliliters (ml) in 1 tablespoon (tbsp). what expression can be used to find the approximate number of milliliters in 3 tbsp?
Answers: 1

Chemistry, 23.06.2019 03:50
How many moles of potassium are needed to react completely with 12.8 moles of magnessium bromide?
Answers: 2

Chemistry, 23.06.2019 10:30
Identify the limiting reactant when 9.65-g h2so4 reacts with 6.10-g of naoh.the equation is h2s04 + 2naoh = 2h2o + na2so4• what is the theoretical yield of na2so4, in grams? • how much of the excess reagent will remain after the reaction has been completed? • if 10.5-g of na2so4 are actually recovered experimentally, what is the percent yield?
Answers: 3

You know the right answer?
The total pressure of an O2-Ar-He gas mixture is 43 atm. If the partial pressure of Ar is 15 atm and...
Questions


English, 03.01.2021 18:40





English, 03.01.2021 18:40

Mathematics, 03.01.2021 18:40


English, 03.01.2021 18:40

Biology, 03.01.2021 18:40

English, 03.01.2021 18:40

Mathematics, 03.01.2021 18:40

Social Studies, 03.01.2021 18:40

Mathematics, 03.01.2021 18:40

Mathematics, 03.01.2021 18:40

Computers and Technology, 03.01.2021 18:40

Mathematics, 03.01.2021 18:40


Business, 03.01.2021 18:40