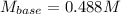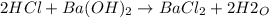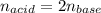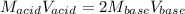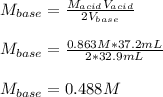Chemistry, 31.05.2021 04:40 aliciaa101
A 32.9-mL sample of Ba(OH)2 is titrated with HCl. If 37.2 mL of 0.863 M HCl is needed to reach the endpoint, what is the concentration (M) of the Ba(OH)2 solution

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
What was the procedure by which case united states vs lopez went to court
Answers: 1

Chemistry, 21.06.2019 20:30
You are to give ampicillin with a recommended dose of 25mg/kg to a child with a mass of 29kg. if stock on hand is 250mg/capsule how many capsules should be given?
Answers: 1

Chemistry, 21.06.2019 22:00
What is driving behind plate tectonics (plate movment)? a) gravity only b) inertia c) convection and gravity d) the sun theres no option for science so i picked chemistry. plz
Answers: 2

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2
You know the right answer?
A 32.9-mL sample of Ba(OH)2 is titrated with HCl. If 37.2 mL of 0.863 M HCl is needed to reach the e...
Questions

History, 09.07.2019 07:00


Mathematics, 09.07.2019 07:00


Mathematics, 09.07.2019 07:00


Mathematics, 09.07.2019 07:00


Mathematics, 09.07.2019 07:00


History, 09.07.2019 07:00

Social Studies, 09.07.2019 07:00



Chemistry, 09.07.2019 07:00





Biology, 09.07.2019 07:00