
Chemistry, 31.05.2021 06:00 Mintfu2580
How many moles of Fe(OH), are produced when 85.0 L of iron(III)
sulfate at a concentration of 0.600 mol/L reacts with excess
NaOH?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 2

Chemistry, 22.06.2019 02:30
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 07:30
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1
You know the right answer?
How many moles of Fe(OH), are produced when 85.0 L of iron(III)
sulfate at a concentration of 0.600...
Questions

Mathematics, 27.05.2021 16:40


Mathematics, 27.05.2021 16:40

English, 27.05.2021 16:40




Spanish, 27.05.2021 16:40



Mathematics, 27.05.2021 16:40


Mathematics, 27.05.2021 16:40

Mathematics, 27.05.2021 16:40

Chemistry, 27.05.2021 16:40



Mathematics, 27.05.2021 16:40


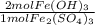 = 102 mol Fe(OH)₃
= 102 mol Fe(OH)₃

