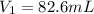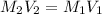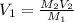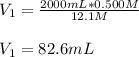Chemistry, 31.05.2021 14:10 kaelynnmarie1135
If hydrochloric acid is obtained commercially at a concentration of 12.1M, how many milliliters of 12.1M HCl(aq) must be used to prepare 2.00x103mL of 0.500M HCL(aq)?

Answers: 3


Another question on Chemistry


Chemistry, 21.06.2019 22:30
For the following, determine the type of reaction and then give products.
Answers: 2

Chemistry, 21.06.2019 23:10
Nitrogen (n), phosphorus (p), and potassium (k) are the main nutrients in plant fertilizers. according to an industry convention, the numbers on the label refer to the mass percents of n, p2o5, and k2o, in that order. calculate the n: p: k ratio of a 30: 10: 10 fertilizer in terms of moles of each element, and express it as x: y: 1.0.
Answers: 1

You know the right answer?
If hydrochloric acid is obtained commercially at a concentration of 12.1M, how many milliliters of 1...
Questions


Chemistry, 04.02.2022 14:00





Mathematics, 04.02.2022 14:00

Computers and Technology, 04.02.2022 14:00

Mathematics, 04.02.2022 14:00

Mathematics, 04.02.2022 14:00

History, 04.02.2022 14:00

English, 04.02.2022 14:00

Mathematics, 04.02.2022 14:00

Mathematics, 04.02.2022 14:00

Chemistry, 04.02.2022 14:00

Social Studies, 04.02.2022 14:00

Chemistry, 04.02.2022 14:00

Mathematics, 04.02.2022 14:00


Biology, 04.02.2022 14:00