Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 19:20
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1

Chemistry, 23.06.2019 04:30
Two liquids are poured into a beaker. after a few seconds, the beaker becomes warm. which of the following best describes this reaction? a. an exothermic reaction b. a decomposition reaction c. an endothermic reaction d. a single-displacement reaction
Answers: 1

Chemistry, 23.06.2019 04:31
How many grams of iron can be made from 16.5 grams of fe2o3
Answers: 1

Chemistry, 23.06.2019 07:00
In order for a high temperature boiler or steam engine to produce superheated water, or steam: the heat source must be greater than 100°c the water must be permitted to evaporate quickly the system must be sealed and become pressurized above atmospheric pressure the vapor pressure must be kept below 760 mm(hg)
Answers: 1
You know the right answer?
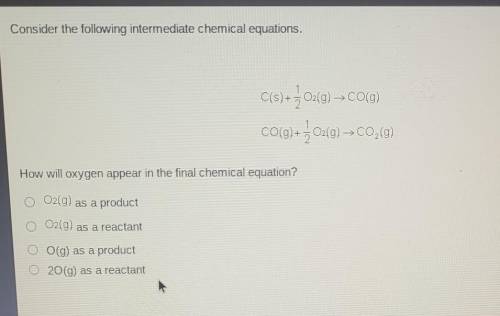
Consider the following intermediate chemical equations.
C(s) + + O2(g) → CO(g) CO(g) + } 02(g) C0,0...
Questions




Physics, 25.02.2022 03:20

Mathematics, 25.02.2022 03:20

Biology, 25.02.2022 03:20

History, 25.02.2022 03:20


Business, 25.02.2022 03:20



Social Studies, 25.02.2022 03:20




Mathematics, 25.02.2022 03:20

Mathematics, 25.02.2022 03:20

Mathematics, 25.02.2022 03:20