
Chemistry, 01.06.2021 01:20 asdf334asdf334
In the acetylene torch, acetylene gas (C2H2) burns in oxygen to produce carbon dioxide water and energy. How many moles of CO2 are formed from the reaction with 1.20 moles of C2H2?
Given the following equation
2C2H2(g) + 502(g) = 4CO2(g) + 2H2O(g)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:00
3. if the dartboard below is used to model an atom, which subatomic particles would be located at z?
Answers: 2

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2
You know the right answer?
In the acetylene torch, acetylene gas (C2H2) burns in oxygen to produce carbon dioxide water and ene...
Questions

Mathematics, 12.10.2020 07:01





English, 12.10.2020 07:01

Mathematics, 12.10.2020 07:01



Mathematics, 12.10.2020 07:01

Mathematics, 12.10.2020 07:01

Mathematics, 12.10.2020 07:01




Mathematics, 12.10.2020 07:01

Biology, 12.10.2020 07:01


Mathematics, 12.10.2020 07:01

Mathematics, 12.10.2020 07:01

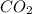 will be formed in the reaction.
will be formed in the reaction.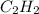 = 1.20 moles
= 1.20 moles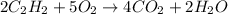
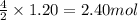 of carbon dioxide
of carbon dioxide

