
Chemistry, 01.06.2021 20:00 twistedhyperboles
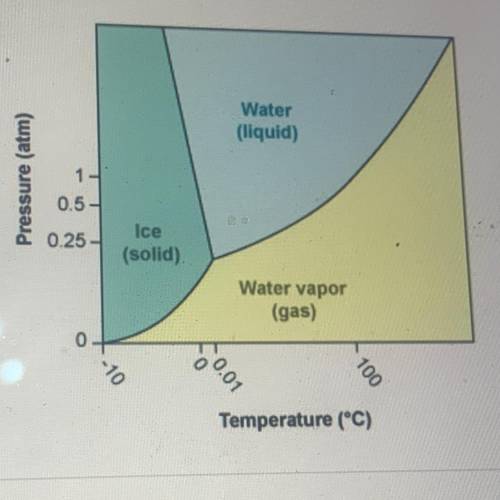
According to the phase diagram for H20, what happens to the phases of
water at 0°C as the pressure is increased from 0 atm to 10 atm?
A. Water changes from a gas to a solid to a liquid
B. Water changes from a liquid to a gas to a solid
C. Water changes from a liquid to a solid to a gas
D. Water changes from a solid to a liquid to a gas

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:50
A100 kmol/h stream that is 97 mole% carbon tetrachloride (ccl4) and 3% carbon disulfide (cs2) is to be recovered from the bottom of a distillation column. the feed to the column is 16 mole% cs2 and 84% ccl4, and 2% of the ccl4 entering the column is contained in the overhead stream leaving the top of the column. calculate the mass and mole fractions of ccl4 in the overhead stream, and determine the molar flow rates of ccl4 and cs2 in the overhead and feed streams. 12. mw_ccla- 153.82; mw_cs2-76.14.
Answers: 3

Chemistry, 22.06.2019 21:00
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1

Chemistry, 22.06.2019 23:00
What is the oxidation state of each individual carbon atom in c2o42−?
Answers: 1

Chemistry, 23.06.2019 00:10
Apropane torch is lit inside a hot air balloon during preflight preparations to inflate the balloon. which condition of the gas remains constant
Answers: 2
You know the right answer?
According to the phase diagram for H20, what happens to the phases of
water at 0°C as the pressure...
Questions


Mathematics, 12.11.2020 19:10

Social Studies, 12.11.2020 19:10






Mathematics, 12.11.2020 19:10



Arts, 12.11.2020 19:10








Computers and Technology, 12.11.2020 19:10



