Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:10
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2

Chemistry, 22.06.2019 17:30
Observation and experimentation have led many scientists to accept a theory about the origin of the universe. this theory is called the big bang theory. scientific evidence collected and observed by scientists around the world suggests that the universe is ever expanding from a hot and dense initial state. what makes this a scientific theory? (2 points)
Answers: 2

Chemistry, 22.06.2019 23:00
What is the mass of naoh that would have to be added to 500 ml of a solution of 0.20 m acetic acid in order to achieve a ph of 5.0?
Answers: 1

Chemistry, 23.06.2019 03:00
Is it safe to take 450mg of diphenhydramine hydrochloride?
Answers: 1
You know the right answer?
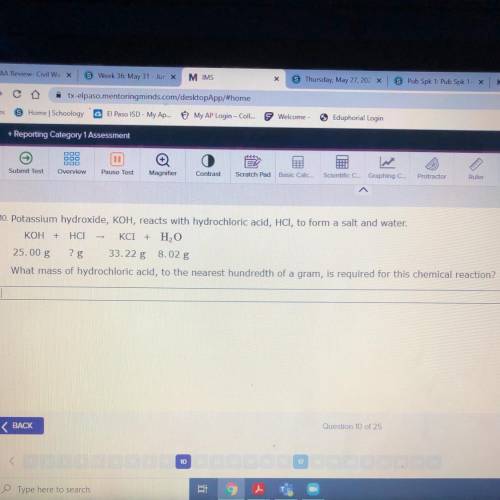
10. Potassium hydroxide, KOH, reacts with hydrochloric acid, HCl, to form a salt and water.
KOH + H...
Questions

Chemistry, 12.12.2020 16:30



Health, 12.12.2020 16:30

History, 12.12.2020 16:30


Mathematics, 12.12.2020 16:30


Mathematics, 12.12.2020 16:30

Mathematics, 12.12.2020 16:30

Mathematics, 12.12.2020 16:30

Mathematics, 12.12.2020 16:30

Biology, 12.12.2020 16:30


Chemistry, 12.12.2020 16:30

Mathematics, 12.12.2020 16:30

German, 12.12.2020 16:30

Mathematics, 12.12.2020 16:30

Mathematics, 12.12.2020 16:30

Biology, 12.12.2020 16:30