
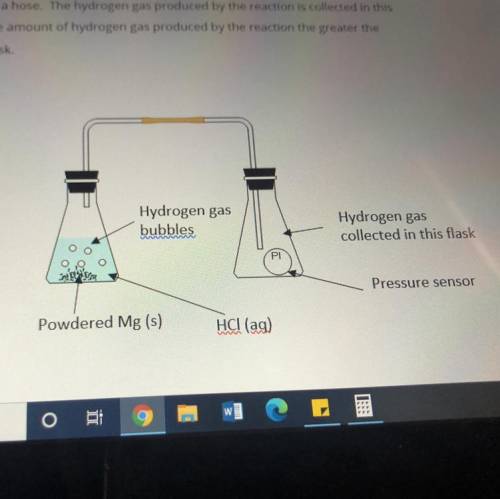
An investigation is conducted into how the mass of magnesium metal reacting with hydrochloric acid affects the amount of hydrogen gas produced.
Masses of 0.10g, 0.20g, 0.30g and 0.40g of powdered Mg metal are reacted with hydrochloric acid(HCl). The conical flask containing the reaction mixture of Mg and HCl is connected to another conical flask with a hose. The hydrogen gas produced by the reaction is collected in this conical flask. The greater the amount of hydrogen gas produced by the reaction the greater the pressure of the gas in the flask.
A) what is the independent variable:
B) what is the dependent variable:
C) write a hypothesis for this investigation:
D) give 2 variables that should have been controlled for this investigation:

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Covalent network solids typically have melting points and boiling points. the chemical formula of a network solid indicates in the molecule.
Answers: 3

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no2]2 ? second order 3/2 order third order zero order none of the listed answers are correct
Answers: 3

Chemistry, 22.06.2019 05:00
Agas can holds 2.0 gal of gasoline. what is this quantity in cubic centimeters?
Answers: 2

Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1
You know the right answer?
An investigation is conducted into how the mass of magnesium metal reacting with hydrochloric acid a...
Questions



Chemistry, 23.03.2021 22:50

Mathematics, 23.03.2021 22:50


Mathematics, 23.03.2021 22:50


Mathematics, 23.03.2021 22:50



Engineering, 23.03.2021 22:50

Mathematics, 23.03.2021 22:50

World Languages, 23.03.2021 22:50

Mathematics, 23.03.2021 22:50


Health, 23.03.2021 22:50

Mathematics, 23.03.2021 22:50





