

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 10:30
Aglow stick contains a glass vial with chemicals. when the glow stick is bent, the vial breaks and the chemicals react to produce a glow. a science student observes that a glow stick kept in the freezer glows for a longer duration than a glow stick kept at room temperature. what conclusion can be drawn based on the observation? be sure to note the outcome and test variables in the conclusion.
Answers: 1

You know the right answer?
200 grams of iron (III) chloride reacts with ammonium carbonate [(NH4)2CO3] in the following equatio...
Questions

Health, 10.07.2019 21:00





Spanish, 10.07.2019 21:00

English, 10.07.2019 21:00

Advanced Placement (AP), 10.07.2019 21:00



Chemistry, 10.07.2019 21:00


Chemistry, 10.07.2019 21:00




Mathematics, 10.07.2019 21:00



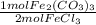 = 0.616 mol Fe₂(CO₃)₃
= 0.616 mol Fe₂(CO₃)₃

