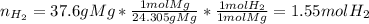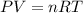Chemistry, 03.06.2021 01:00 piratesfc02
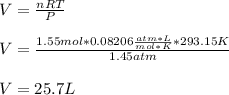
What volume of hydrogen will produced at 1.45 atm and a tempeture of 20C by the reaction of 37.6g of magnesium 1Mg+2H2O--> Mg(OH)2+H2

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Electrons are extremely important to what area of technology? a) anti-aging research b) household product development c) electronics d) drug discovery
Answers: 3

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

Chemistry, 22.06.2019 19:40
What is the wavelength of a 3*10^12 hz infrared wave a 3*10^20m b 1* 10^4m c 3*10^-3m d 1*10^-4 m
Answers: 1

Chemistry, 22.06.2019 21:30
If 22.5 of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. what is the new volume?
Answers: 1
You know the right answer?
What volume of hydrogen will produced at 1.45 atm and a tempeture of 20C by the reaction of 37.6g of...
Questions

Mathematics, 13.03.2021 03:10

Chemistry, 13.03.2021 03:10

Mathematics, 13.03.2021 03:10



Mathematics, 13.03.2021 03:10

Mathematics, 13.03.2021 03:10

Social Studies, 13.03.2021 03:10

English, 13.03.2021 03:10

Mathematics, 13.03.2021 03:10

Geography, 13.03.2021 03:10

Mathematics, 13.03.2021 03:10

Social Studies, 13.03.2021 03:10

Mathematics, 13.03.2021 03:10

Mathematics, 13.03.2021 03:10


Geography, 13.03.2021 03:10

Mathematics, 13.03.2021 03:10

Chemistry, 13.03.2021 03:10