
Chemistry, 03.06.2021 01:10 robert7248
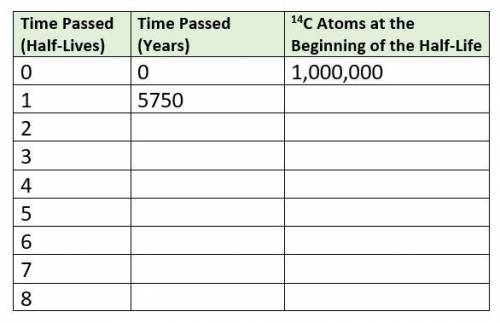
1. Use the table above. Scientists find a piece of wood that is thought to be from an ancient fire circle. They find that the wood contains an amount of carbon-14 (14C) that is approximately 1/16 of the current atmospheric 14C levels. 14C has a t1/2 of 5,750 years. (5 pts)How many half lives is represented by 1/16? How many years ago was the tree chopped down to be used for firewood?If you started with 1,000,000 carbon-14 atoms, how many atoms would remain in the wood?
Please help, don't send some obscure link, thanks so much!
I attached the chart

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
An electrons position cannot be known precisely only it's probability of being in a certain location can be known
Answers: 1

Chemistry, 23.06.2019 05:40
Why is it incorrect to balance a chemical equation by changing the subscripts? explain.
Answers: 2

Chemistry, 23.06.2019 06:40
The combustion of methane, ch4, releases 890.4kj/mol. that is, when one mole of methane is burned,890.4 kj are given off to the surroundings. this meansthat the products have 890.4 kj less than the reactants.thus, ah for the reaction = - 890.4 kj. a negative symbolforah indicates an exothermic reaction.ch (g) + 20 (g)> co2 (g) + 2 h0 (1); ah = - 890.4 kga) how much energy is given off when 2.00 mol of ch,are burned? b) how much energy is released when 22.4g of ch. areburned?
Answers: 1

Chemistry, 23.06.2019 11:30
A) equal lines b) parallel lines c) perpendicular lines d) none of the above
Answers: 1
You know the right answer?
1. Use the table above. Scientists find a piece of wood that is thought to be from an ancient fire c...
Questions


Mathematics, 16.07.2019 22:40






Chemistry, 16.07.2019 22:40





Mathematics, 16.07.2019 22:40

Biology, 16.07.2019 22:40

Mathematics, 16.07.2019 22:40




History, 16.07.2019 22:40



