Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which describes interactions between substances and stomata during photosynthesis? check all that apply. oxygen enters stomata. oxygen is released through stomata. carbon dioxide enters stomata. carbon dioxide is released through stomata. hydrogen enters stomata. hydrogen is released through stomata.
Answers: 1

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2

Chemistry, 23.06.2019 03:00
Use the half-reactions of the reaction au(oh)3 + hi -> au +i2 +h2o to answer the questions
Answers: 1

Chemistry, 23.06.2019 10:00
Lord kelvin described the concept of absolute zero temperature and the laws relating to the change of thermal energy during chemical reactions what type of chemist would he be considered today
Answers: 1
You know the right answer?
Someone pls help me ::/:/
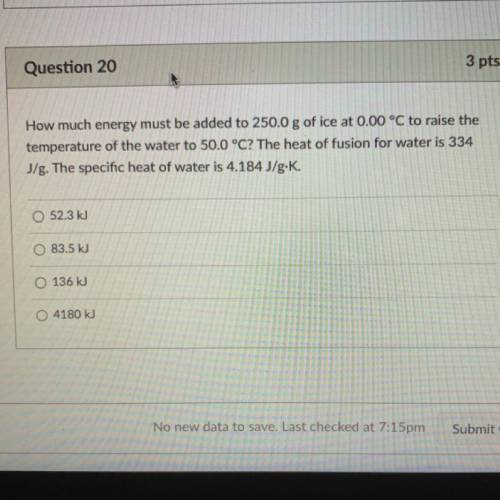
How much energy must be added to 250.0 g of ice at 0.00 °C to raise the
Questions



History, 02.06.2021 05:00




Mathematics, 02.06.2021 05:00

History, 02.06.2021 05:00


Chemistry, 02.06.2021 05:00

Biology, 02.06.2021 05:00

Mathematics, 02.06.2021 05:00

Mathematics, 02.06.2021 05:00



Arts, 02.06.2021 05:00


Mathematics, 02.06.2021 05:00


Mathematics, 02.06.2021 05:00