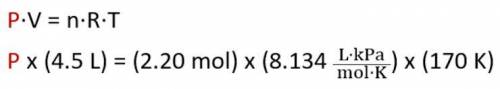Answers: 3


Another question on Chemistry



Chemistry, 23.06.2019 09:10
Complete the following radioactive decay problem. tan+on-? c+th
Answers: 1

Chemistry, 23.06.2019 16:00
Why is it important for scientists to replicate each other’s experiments? to determine if important scientific results are repeatable to the research of other scientists to determine if slight alterations in the experiment can affect the result to further their own research
Answers: 2
You know the right answer?
What is the pressure of 2.20 mol of a gas stored in a 4.5 L container at a temperature of 170 K? (Us...
Questions

Mathematics, 14.12.2020 01:30

Mathematics, 14.12.2020 01:30

Mathematics, 14.12.2020 01:30


Mathematics, 14.12.2020 01:30

Business, 14.12.2020 01:30


Mathematics, 14.12.2020 01:30

English, 14.12.2020 01:30


English, 14.12.2020 01:30

Mathematics, 14.12.2020 01:30

Computers and Technology, 14.12.2020 01:30



Mathematics, 14.12.2020 01:30

Mathematics, 14.12.2020 01:30


Mathematics, 14.12.2020 01:30