1. Specific Heat Capacity.
A. Heat on concrete
The specific heat capacity of concrete is 0.88...

Chemistry, 03.06.2021 16:50 genyjoannerubiera
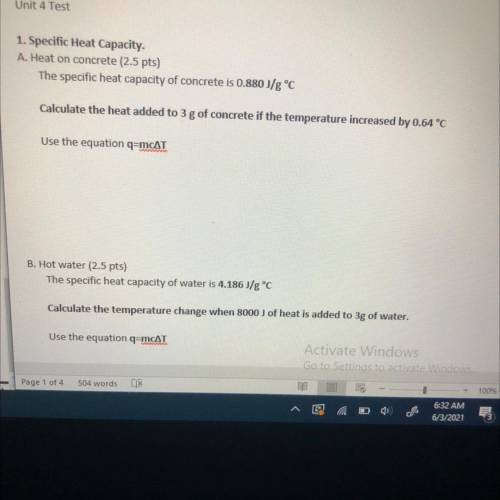
1. Specific Heat Capacity.
A. Heat on concrete
The specific heat capacity of concrete is 0.880 J/g °C
Calculate the heat added to 3 g of concrete if the temperature increased by 0.64 °C
Use the equation q=mcat
B. Hot water
The specific heat capacity of water is 4.186J/g °C
Calculate the temperature change when 8000 J of heat is added to 3g of water,
Use the equation q=mcat

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
How did planetesmals form planets? a. they broke apart into smaller chunks.b. they collided and stuck together.c. they cooled and pulled ice together.d. they began to rotate.
Answers: 1

Chemistry, 22.06.2019 05:00
Agas can holds 2.0 gal of gasoline. what is this quantity in cubic centimeters?
Answers: 2

Chemistry, 22.06.2019 11:00
The number to the right of an element's symbol (ex. c-12) identifies the of an isotope.
Answers: 1

You know the right answer?
Questions


English, 24.09.2020 06:01

Geography, 24.09.2020 06:01

Social Studies, 24.09.2020 06:01







Mathematics, 24.09.2020 06:01


English, 24.09.2020 06:01

Chemistry, 24.09.2020 06:01

English, 24.09.2020 06:01

Computers and Technology, 24.09.2020 06:01




Mathematics, 24.09.2020 06:01



