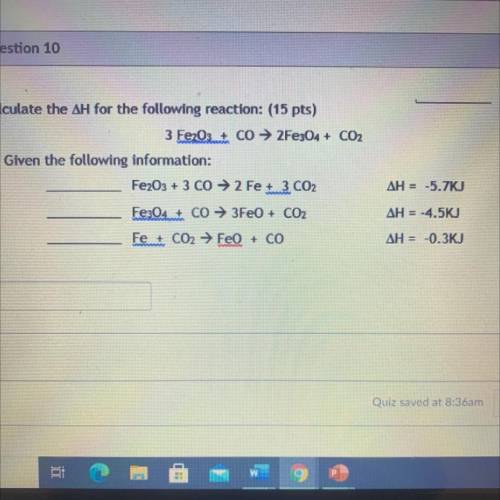
Calculate the ΔH for the following reaction: (15 pts)
3 Fe2O3+ Co → 2Fe304 + CO2
Given the fo...

Chemistry, 03.06.2021 17:00 hjeffrey168
Calculate the ΔH for the following reaction: (15 pts)
3 Fe2O3+ Co → 2Fe304 + CO2
Given the following information:
Fe2O3 + 3 CO → 2 Fe 3 CO2
ΔH = -5.7KJ
Fe3O4 + CO → 3FeO + CO2
ΔH = -4.5KJ
Fe + CO2 → FeO + CO
ΔH = -0.3KJ

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1


Chemistry, 22.06.2019 10:20
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3

Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2
You know the right answer?
Questions



Mathematics, 09.04.2020 15:45

Chemistry, 09.04.2020 15:55

Mathematics, 09.04.2020 15:55

Physics, 09.04.2020 15:55








Social Studies, 09.04.2020 15:55

History, 09.04.2020 15:55


Mathematics, 09.04.2020 15:55



Mathematics, 09.04.2020 15:55



