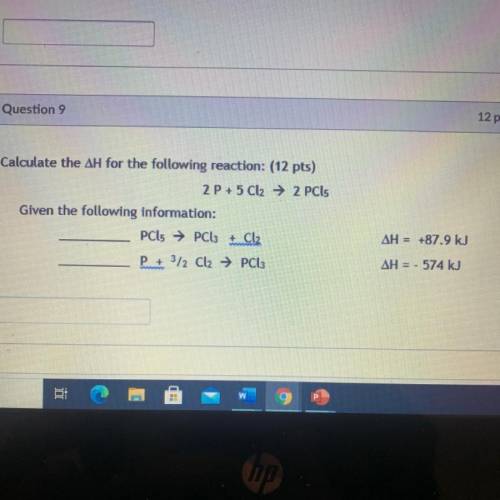
Calculate the ΔH for the following reaction:
2 P + 5 Cl2 → 2 PCL5
Given the following informa...


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:40
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3

Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 23.06.2019 02:00
Which of these is a density dependent factor? a. epidemic b. earthquake c. drought d. hurricane
Answers: 2

You know the right answer?
Questions


Mathematics, 26.10.2021 14:00




Mathematics, 26.10.2021 14:00

Geography, 26.10.2021 14:00


History, 26.10.2021 14:00

English, 26.10.2021 14:00

Physics, 26.10.2021 14:00


History, 26.10.2021 14:00



English, 26.10.2021 14:00