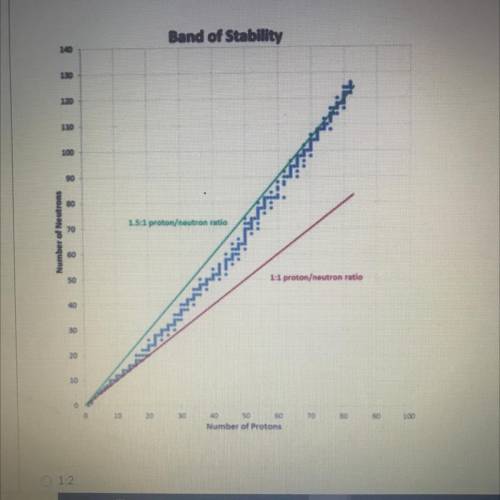
Ahh sorry i need help
A. 1:2
B. 1.5:1
C. 1:1
...

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:20
1. suppose a reaction mixture, when diluted with water, afforded 300 ml of an aqueous solution of 30 g of the reaction product malononitrile [ch2(cn)2], which is to be isolated by extraction with ether. the solubility of malononitrile in ether at room temperature is 20.0 g/100 ml, and in water is 13.3 g/100 ml. what weight of malononitrile would be recovered by extraction with (a) three 100-ml portions of ether and (b) one 300-ml portion of ether? suggestion: for each extraction, let x equal the weight extracted into the ether layer. in part (a), the concentration in the ether layer is x/100 and in the water layer is (30 x)/300; the ratio of these quantities is equal to k 20/13.3.
Answers: 2

Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 22.06.2019 08:30
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2
You know the right answer?
Questions




Computers and Technology, 12.03.2021 15:10


Computers and Technology, 12.03.2021 15:10









Computers and Technology, 12.03.2021 15:10