
Chemistry, 03.06.2021 18:00 hjeffrey168
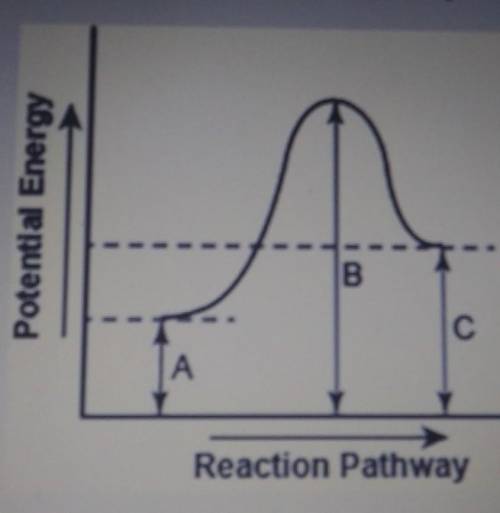
The diagram below shows the potential energy changes for a reaction pathway.
Part 1: Does the diagram illustrate an enothermic or an exothermic reaction? Give reasons to support your answer.
Part 2: Describe how you can determine the total change in ethalpy and activation energy from the diagram and if each is positive or negative.
Brainliest if you give the definition of endo and exothermic reactions, and how you know the change in enthalpy even if you put nothing else.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
An empty fuel tank can still contain and therefore can be even more dangerous than one full of liquid fuel.
Answers: 1

Chemistry, 22.06.2019 09:00
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2

Chemistry, 22.06.2019 09:00
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2
You know the right answer?
The diagram below shows the potential energy changes for a reaction pathway.
Part 1: Does the diagr...
Questions

History, 26.10.2020 22:00



Mathematics, 26.10.2020 22:00

History, 26.10.2020 22:00




Mathematics, 26.10.2020 22:00



English, 26.10.2020 22:00


Geography, 26.10.2020 22:00

Mathematics, 26.10.2020 22:00


Mathematics, 26.10.2020 22:00

Mathematics, 26.10.2020 22:00


Mathematics, 26.10.2020 22:00



