
Northern cod produce proteins that protect their cells from damage caused by subzero temperatures. Measurements of the osmotic pressure for two "antifreeze" proteins at 18°C yielded the data listed below. Use this information to calculate the molar mass for each of the proteins. Assume these proteins are nonelectrolytes and use the value i = 1.
Required:
If a 54.1 mg sample of protein A in 1.5 mL of water has an osmotic pressure of 0.285 atm, what is the molar mass of protein A?

Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 04:40
[01.07]what is the answer to the problem: 101 g + 25.01 g + 5.05 g? 131.06 g 131.1 g 131 g 130 g
Answers: 1

Chemistry, 23.06.2019 05:30
Calculate the temperature rise when 0.2g of propane is used to heat 400cm cubed of water.
Answers: 3

Chemistry, 23.06.2019 11:20
Which of the following is a pure substance? airbloodcopperwood
Answers: 2

Chemistry, 23.06.2019 13:00
Using the periodic table complete the table to describe each atom type in your answers
Answers: 1
You know the right answer?
Northern cod produce proteins that protect their cells from damage caused by subzero temperatures. M...
Questions




Chemistry, 19.11.2020 22:30


Mathematics, 19.11.2020 22:30

Chemistry, 19.11.2020 22:30



Mathematics, 19.11.2020 22:30

Mathematics, 19.11.2020 22:30

English, 19.11.2020 22:30


Mathematics, 19.11.2020 22:30


Social Studies, 19.11.2020 22:30


History, 19.11.2020 22:30

Social Studies, 19.11.2020 22:30

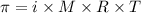
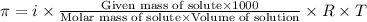 .....(1)
.....(1)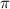 = osmotic pressure = 0.285 atm
= osmotic pressure = 0.285 atm![18^oC=[18+273]=291K](/tpl/images/1361/3277/7621e.png)



