Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:30
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1

Chemistry, 22.06.2019 18:30
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1

Chemistry, 22.06.2019 20:00
How are the terms group and period used on the periodic table
Answers: 1

Chemistry, 22.06.2019 21:00
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1
You know the right answer?
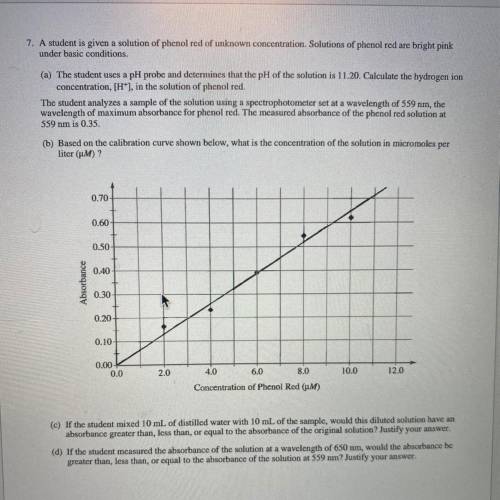
(c) If the student mixed 10 mL of distilled water with 10 mL of the sample, would this diluted solut...
Questions

Mathematics, 04.10.2020 16:01

Mathematics, 04.10.2020 16:01

Spanish, 04.10.2020 16:01


Geography, 04.10.2020 16:01




Mathematics, 04.10.2020 16:01

History, 04.10.2020 16:01

Social Studies, 04.10.2020 16:01


SAT, 04.10.2020 16:01

History, 04.10.2020 16:01

Mathematics, 04.10.2020 16:01

History, 04.10.2020 16:01