
Chemistry, 04.06.2021 03:50 toribrown3773
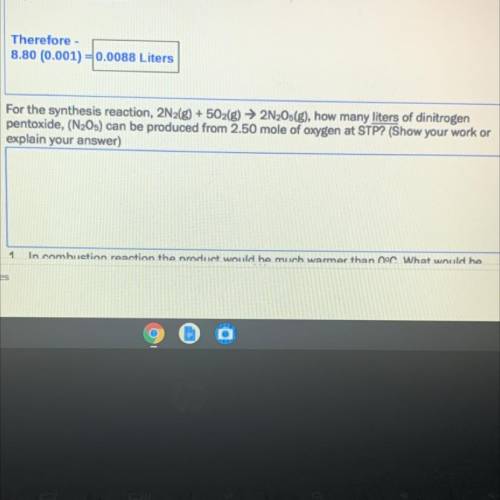
For the synthesis reaction, 2N2(g) + 5O2(g) → 2N2Os(g), how many liters of dinitrogen
pentoxide, (N205) can be produced from 2.50 mole of oxygen at STP? (Show your work or
explain your answer)
*SEE PICTURE FOR BETTER UNDERSTANDING*

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:20
Calculate the enthalpy of the following reaction: 4 b (s) + 3 o2 (g) → 2 b2o3 (s) given the following pertinent information: (a) b2o3 (s) + 3 h2o (g) → 3 o2 (g) + b2h6 (g), δhoa = +2035 kj (b) 2 b (s) + 3 h2 (g) → b2h6 (g), δhob = +36 kj (c) h2 (g) + latex: \frac{1}{2} 1 2 o2 (g) → h2o (l), δhoc = −285 kj (d) h2o (l) → h2o (g), δhod = +44 kj
Answers: 3

Chemistry, 22.06.2019 23:30
The density of benzene at 15 °c is 0.8787 g/ml. calculate the mass of 0.1500 l of benzene at this temperature. enter your answer in terms of grams
Answers: 2


Chemistry, 23.06.2019 01:30
Which of the following statements is true about energy quantization at the atomic level? electrons in the outermost orbits are the most stable. electrons in all the orbits around the nucleus have the same amount of energy. electrons in the orbit closest to the nucleus have the least amount of energy. electrons absorb or release the same amount of energy independent of the energy levels.
Answers: 1
You know the right answer?
For the synthesis reaction, 2N2(g) + 5O2(g) → 2N2Os(g), how many liters of dinitrogen
pentoxide, (N...
Questions

Mathematics, 13.11.2019 10:31

English, 13.11.2019 10:31

English, 13.11.2019 10:31



Chemistry, 13.11.2019 10:31



English, 13.11.2019 10:31


Health, 13.11.2019 10:31


Business, 13.11.2019 10:31

Health, 13.11.2019 10:31



Biology, 13.11.2019 10:31

Mathematics, 13.11.2019 10:31

Mathematics, 13.11.2019 10:31



