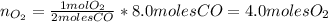Chemistry, 04.06.2021 04:40 taleiayarbough9783
The balanced equation below represents the reaction between carbon and hydrogen to produce
ethane.
2C(s) + 3H2(g) → C2H6(g) + energy
Determine the number of moles of O2(g) needed to completely react with 8.0 moles of CO(g).
Please help!!!


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 22:30
What if it is did darwin used to support his theory of evolution
Answers: 1

Chemistry, 23.06.2019 00:00
What is the empirical formula of a compound that is 50.7% antimony and 49.3% selenium ?
Answers: 2

Chemistry, 23.06.2019 00:30
How can you write e method for the experiment of separating sand from water by filtration process? 1-materials 2-steps 3-conclusion also the same for the separating process of water and salt by filtration or distillation. quick because i need to finish my hw
Answers: 2

Chemistry, 23.06.2019 02:30
Which statement best describes the liquid state of matter? a. it has definite shape but indefinite volume. b. it has definite shape and definite volume. c. it has indefinite shape and indefinite volume. d. it has indefinite shape but definite volume.
Answers: 1
You know the right answer?
The balanced equation below represents the reaction between carbon and hydrogen to produce
ethane.<...
Questions


History, 07.05.2021 22:10

Mathematics, 07.05.2021 22:10



Mathematics, 07.05.2021 22:10


Mathematics, 07.05.2021 22:20

Mathematics, 07.05.2021 22:20


Mathematics, 07.05.2021 22:20



Mathematics, 07.05.2021 22:20


English, 07.05.2021 22:20



Mathematics, 07.05.2021 22:20

Mathematics, 07.05.2021 22:20