
Chemistry, 04.06.2021 06:50 donnafranks2003
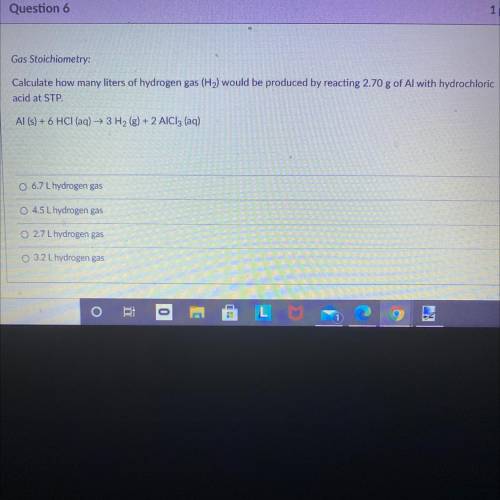
Gas Stoichiometry:
Calculate how many liters of hydrogen gas (H2) would be produced by reacting 2.70 g of Al with hydrochloric
acid at STP.
Al (s) + 6 HCl (aq) + 3H2(g) + 2 AlCl3 (aq)
6.7 L hydrogen gas
O 4.5 L hydrogen gas
O 2.7 L hydrogen gas
3.2 L hydrogen gas

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
What layer of the atmosphere is directly above the troposphere?
Answers: 1

Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2

Chemistry, 22.06.2019 22:20
How do cfcs cause ozone depletion? how do cfcs cause ozone depletion? ultraviolet radiation breaks down cfcs, molecules containing chlorine. chlorine then breaks one oxygen atom away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation breaks down cfcs, molecules containing chlorine. chlorine then breaks two oxygen atoms away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation creates cfcs, molecules containing chlorine. chlorine then breaks two oxygen atoms away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation creates cfcs, molecules containing chlorine. chlorine then breaks one oxygen atom away from ozone, leaving behind a paired oxygen molecule.
Answers: 2

Chemistry, 23.06.2019 04:30
Two liquids are poured into a beaker. after a few seconds, the beaker becomes warm. which of the following best describes this reaction? a. an exothermic reaction b. a decomposition reaction c. an endothermic reaction d. a single-displacement reaction
Answers: 1
You know the right answer?
Gas Stoichiometry:
Calculate how many liters of hydrogen gas (H2) would be produced by reacting 2.7...
Questions


Spanish, 26.08.2019 20:00

Mathematics, 26.08.2019 20:00


Arts, 26.08.2019 20:00

Mathematics, 26.08.2019 20:00

Mathematics, 26.08.2019 20:00



Mathematics, 26.08.2019 20:00



History, 26.08.2019 20:00


English, 26.08.2019 20:00

History, 26.08.2019 20:00


Mathematics, 26.08.2019 20:00

English, 26.08.2019 20:00




