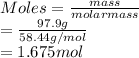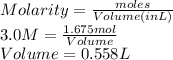PLEASE HELP Let’s say a colleague working in the lab needs to create a solution containing 97.9 grams of NaCl. If she has a 3.0 M stock solution of NaCl dissolved in water, how many liters of the stock solution would she need to have 97.9 grams NaCl? Remember the molar mass of NaCl is 58.44 g/mol.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Which statement describes covalent bases? they have hydroxide ions. they produce hydrogen ions. they are often amines. they are named the same as ionic compounds.
Answers: 3

Chemistry, 22.06.2019 07:00
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.50 grams of aluminum foil in a solution containing 0.75 grams of copper (ii) chloride. a single replacement reaction takes place. (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction? a) approximately 0.36 grams, because copper (ii) chloride acts as a limiting reactant b) approximately 1.8 grams, because copper (ii) chloride acts as a limiting reactant c) approximately 0.36 grams, because aluminum acts as a limiting reactant d) approximately 1.8 grams, because aluminum acts as a limiting reactant
Answers: 3


Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1
You know the right answer?
PLEASE HELP
Let’s say a colleague working in the lab needs to create a solution containing 97.9 gra...
Questions



History, 17.07.2019 02:00

History, 17.07.2019 02:00

Mathematics, 17.07.2019 02:00


English, 17.07.2019 02:00


Social Studies, 17.07.2019 02:00


History, 17.07.2019 02:00


History, 17.07.2019 02:00



Physics, 17.07.2019 02:00

Mathematics, 17.07.2019 02:00

History, 17.07.2019 02:00

Chemistry, 17.07.2019 02:00