
Chemistry, 04.06.2021 18:00 gissellesolorza
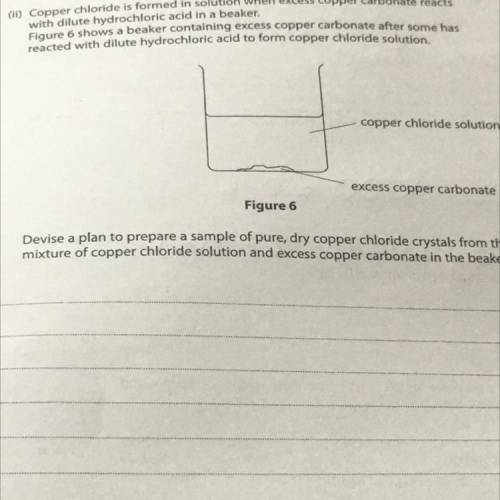
(11) Copper chloride is formed in solution when excess copper carbonate reacts
with dilute hydrochloric acid in a beaker.
Figure 6 shows a beaker containing excess copper carbonate after some has
reacted with dilute hydrochloric acid to form copper chloride solution.
DO NOT WRITE IN THIS AREA
DO NOT WRITE IN THIS AREN
copper chloride solution
excess copper carbonate
Figure 6
Devise a plan to prepare a sample of pure, dry copper chloride crystals from the
mixture of copper chloride solution and excess copper carbonate in the beaker.
DO NOT
THIS AREA
A

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
In which phase(s) do the molecules take the shape of the container?
Answers: 1

Chemistry, 22.06.2019 07:00
How many moles are in 7.2 x 10^23 carbon molecules? (*round to the nearest hundredth and include the unit "mol c" after your number) question 6 options:
Answers: 2

Chemistry, 22.06.2019 08:00
What are the similarities of physical and chemical change ?
Answers: 1

Chemistry, 22.06.2019 19:50
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3
You know the right answer?
(11) Copper chloride is formed in solution when excess copper carbonate reacts
with dilute hydrochl...
Questions





History, 11.07.2019 15:00





Computers and Technology, 11.07.2019 15:00



History, 11.07.2019 15:00







Computers and Technology, 11.07.2019 15:00



