
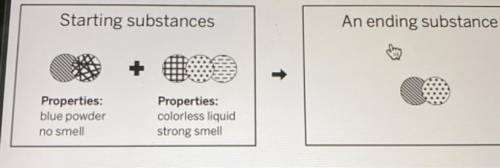
WILL GIVE BRAINIEST HELP ASAP chemist mixed two substances together: a blue powder with no smell and a colorless liquid with a strong smell. Their repeating groups of atoms are shown above on the left. After they were mixed, the chemist analyzed the results and found two substances. One ending substance had the repeating group of atoms shown above on the right. Is the ending substance the same substance as the blue powder? What happened to the atoms of the starting substances when the ending substances formed? Be sure to explain your answers to both of these questions.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical solutions?
Answers: 1

Chemistry, 23.06.2019 02:00
Butane gas reacts with oxygen gas to give carbon dioxide gas and water vapor (gas). if you mix butane and oxygen in the correct stoichiometric ratio, and if the total pressure of the mixture is 390 mmhg, what is the pressure (in mmhg) of water vapor after the reaction is completed (temperature and volume do not change).
Answers: 2

Chemistry, 23.06.2019 08:30
Sand is more likely than shale to preserve fossils. true false
Answers: 2

Chemistry, 23.06.2019 20:30
I'm a tad bit confused on how to name compounds with molar mass and was wondering what was the difference between the formulas dealing with covalent and iconic if someone could pls explain these directions that would be great
Answers: 2
You know the right answer?
WILL GIVE BRAINIEST HELP ASAP chemist mixed two substances together: a blue powder with no smell and...
Questions

Mathematics, 08.11.2020 01:50


English, 08.11.2020 01:50

Mathematics, 08.11.2020 01:50





Business, 08.11.2020 01:50

Mathematics, 08.11.2020 01:50



Mathematics, 08.11.2020 01:50


English, 08.11.2020 01:50

Social Studies, 08.11.2020 01:50

Physics, 08.11.2020 01:50



Computers and Technology, 08.11.2020 02:00



