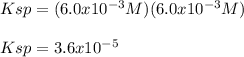Chemistry, 05.06.2021 03:40 hogwartsalicia
Solid strontium chromate, SrCrO4, dissolves into its respective ions at 25°C. Suppose that in a particular solution, [Sr^2+]=6.0x10^-3M. Find the value of Ksp.
A) 3.6 x 10^-5
B) 6.0 x 10^-3
C) 3.6 x 10^-2
D) 7.7 x 10^-2

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:00
19. at high pressures, how does the volume of a real gas compare with the volume of an ideal gas under the same conditions, and why? eman- it is much less because real gas partides are not moving. there is no difference because the gas laws are always obeyed. it is much less because at high pressures the temperature drops. it is much greater because real gas partides take up space.
Answers: 1

Chemistry, 21.06.2019 22:00
Which solution is an example of a nonelectrolyte? a. iodine in hexane b. sodium nitrate in waterc. acetic acid in waterd. hydrogen chloride in water
Answers: 2

Chemistry, 22.06.2019 02:30
Asa choose the correct set of reaction coefficients to properly balance the following chemical equation according to the law of conservation of mass: __s8 + __o2 ==> __so2 1, 1, 8 1, 8, 1 1, 8, 8 8, 1, 1
Answers: 1

Chemistry, 22.06.2019 14:50
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3
You know the right answer?
Solid strontium chromate, SrCrO4, dissolves into its respective ions at 25°C. Suppose that in a part...
Questions



English, 23.11.2020 20:30

Business, 23.11.2020 20:30






English, 23.11.2020 20:30



Mathematics, 23.11.2020 20:30


Mathematics, 23.11.2020 20:30

Health, 23.11.2020 20:30

Mathematics, 23.11.2020 20:30



Mathematics, 23.11.2020 20:30

![Ksp=[Sr^{2+}][CrO_4^{2-}]](/tpl/images/1363/6886/12f99.png)