Chemistry, 05.06.2021 14:00 winterblanco
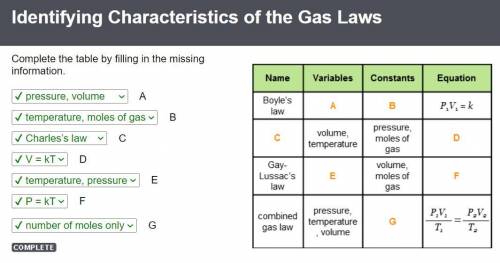
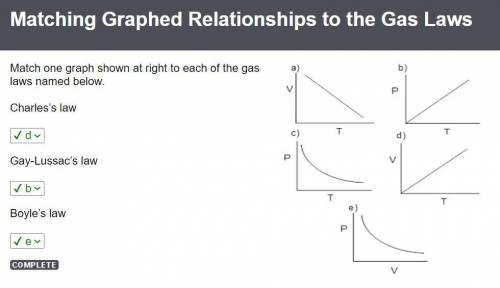
If you decreased the volume of a sample of gas by a factor of three while maintaining a constant pressure, how would the absolute temperature of the gas be affected?
A gas at 300 K and 4.0 atm is moved to a new location with a temperature of 250 K. The volume changes from 5.5 L to 2.0 L. What is the pressure of the gas at the new location?
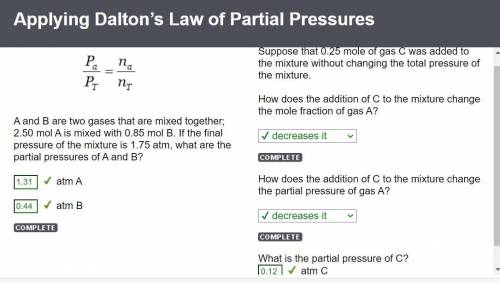
What is the partial pressure of 0.50 mol Ne gas combined with 1.20 mol Kr gas at a final pressure of 730 torr?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 12:50
5. how can you decrease the pressure of a gas in a container without changing the volume of the gas?
Answers: 1

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 1

Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1

Chemistry, 23.06.2019 01:30
List and describe the neurological effects of the vocs and other air pollutants,as described by dr.theo colborn
Answers: 2
You know the right answer?
If you decreased the volume of a sample of gas by a factor of three while maintaining a constant pre...
Questions


History, 02.06.2021 19:40



Mathematics, 02.06.2021 19:40


English, 02.06.2021 19:40

English, 02.06.2021 19:40

Mathematics, 02.06.2021 19:40


Mathematics, 02.06.2021 19:40


Mathematics, 02.06.2021 19:40


Mathematics, 02.06.2021 19:40

Chemistry, 02.06.2021 19:40


Mathematics, 02.06.2021 19:40