
Chemistry, 05.06.2021 22:50 Kaysofine11icloudcom
What is the limiting reactant in a reaction where 10.0 mol of iron is treated with 12.0 mol of bromine? The product that forms is FeBr3. First, write and balance the chemical equation. Next, calculate the moles of FeBr3 that can be made from 10.0 mol of Fe. Then calculate the moles of FeBr3 that can be made from 12.0 mol of bromine. The smaller amount of FeBr3 reveals the limiting reactant.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 12:50
5. how can you decrease the pressure of a gas in a container without changing the volume of the gas?
Answers: 1


Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 23:50
Which scientists contributed to the determination of how cfcs in clouds in the upper atmosphere could destroy ozone molecules
Answers: 1
You know the right answer?
What is the limiting reactant in a reaction where 10.0 mol of iron is treated with 12.0 mol of bromi...
Questions


Geography, 06.02.2021 02:30

Social Studies, 06.02.2021 02:30

Computers and Technology, 06.02.2021 02:30

Mathematics, 06.02.2021 02:30


History, 06.02.2021 02:30



History, 06.02.2021 02:30

Mathematics, 06.02.2021 02:30

Mathematics, 06.02.2021 02:30

Engineering, 06.02.2021 02:30





Mathematics, 06.02.2021 02:30

Chemistry, 06.02.2021 02:30

Mathematics, 06.02.2021 02:30

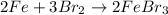
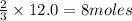 of iron
of iron

