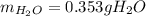Chemistry, 06.06.2021 01:30 alannaswitzer
Calculate the mass of water vapor that is produced by the reaction: 
1.4 g of CO2 and 2.2 g of KOH in the reaction: CO2 + 2KOH → K2CO3 + H20
Please show work, will give brainliest

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:20
Which is true of chemicals? a. things containing chemicals always cost a lot of money. b. chemicals are never dangerous. c. chemicals are in many substances in a home. d. chemicals are rarely found on earth.
Answers: 1

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1

Chemistry, 22.06.2019 19:00
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2

Chemistry, 22.06.2019 22:30
Essay-alternative energy sources research sources of energy that are being developed. write a report of 350-400 words discussing the information you learned concerning the development of various energy sources and the impact that you think they will have on your life. include sources cited at the end of your report using the mla format. follow the rubric guidelines. note that wikipedia is not an appropriate resource for a research paper. worth 99
Answers: 3
You know the right answer?
Calculate the mass of water vapor that is produced by the reaction: 
1.4 g of CO2 and 2.2 g of KOH...
Questions

Mathematics, 16.02.2021 14:00

Mathematics, 16.02.2021 14:00

Mathematics, 16.02.2021 14:00

Geography, 16.02.2021 14:00

Mathematics, 16.02.2021 14:00

Health, 16.02.2021 14:00

Social Studies, 16.02.2021 14:00

Biology, 16.02.2021 14:00

Mathematics, 16.02.2021 14:00


English, 16.02.2021 14:00

Health, 16.02.2021 14:00


Physics, 16.02.2021 14:00



History, 16.02.2021 14:00

History, 16.02.2021 14:00

English, 16.02.2021 14:00